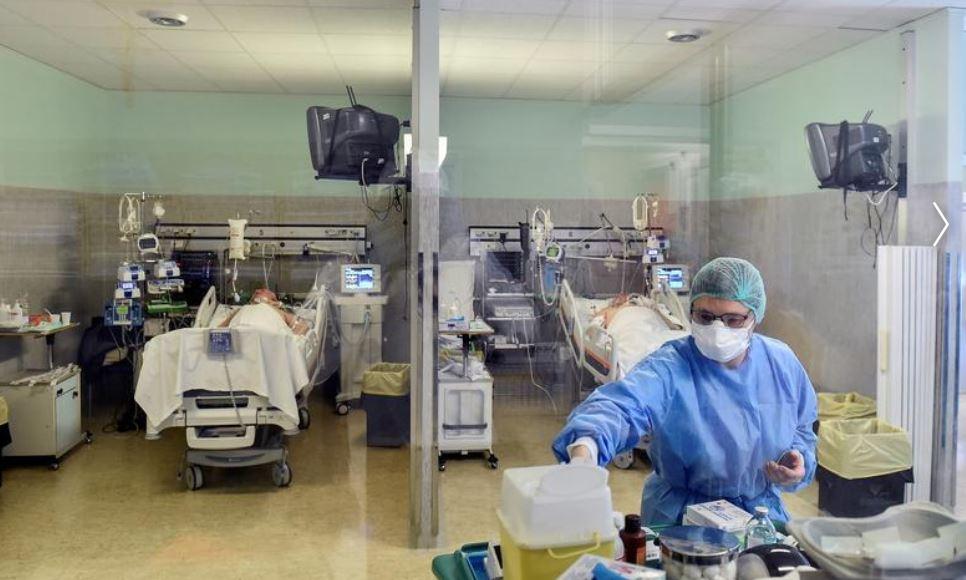
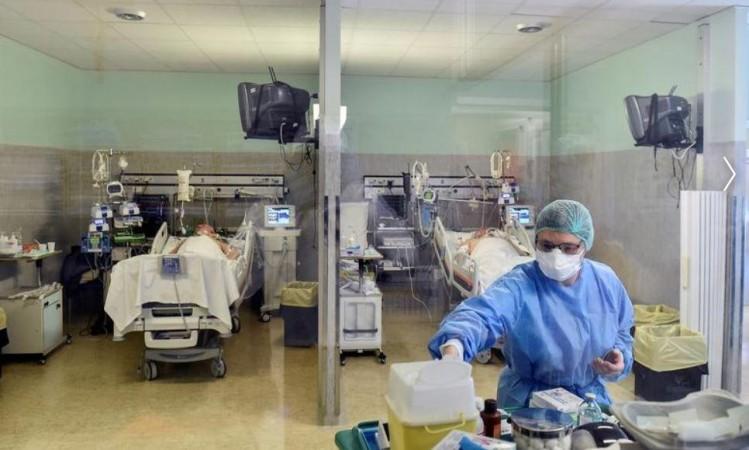
A good vaccine usually will take at minimum 18 months, ahead of it could be rolled out for generation soon after fast-tracking all the mandatory regulatory approvals, reported N.K. Ganguly, previous director standard of Indian Council of Healthcare Investigate (ICMR).
It is envisioned that India’s first indigenous COVID-19 vaccine, Covaxin might be introduced on August 15 beneath a rapid-track mechanism. In accordance to the government’s top health-related exploration physique, a dozen institutes have been selected for clinical trials of the indigenous COVID-19 vaccine (BBV152 COVID vaccine). Ganguly mentioned the strain of the virus was provided to BBIL in May perhaps close, and in July, human trials have been scheduled.
Ex-ICMR Chief on COVID-19 vaccine
Elaborating on the lengthy approach included in vaccine progress, he insisted that soon after investing numerous months and infusing a good deal of resources, the uncertainty proceeds to linger. “It may possibly be tough to say regardless of whether the vaccine is effective or not”, he additional.
The ICMR in a letter reported, “It is envisaged to launch the vaccine for public wellness use most current by August 15, right after completion of all medical trials. BBIL (Bharat Biotech International Constrained) is operating expeditiously to fulfill the target, nevertheless the final end result will depend on the cooperation of all scientific demo web sites associated in this challenge.”
Before the vaccine is examined on people, challenge studies are accomplished on mice and monkey, then a toxicology report is organized, to look at if the formulated vaccine provides hurt to cells, and it will take at the very least a few to 4 months to finalize this report, reported Ganguly. Immediately after this phase, the vaccine is tested on two rodents and a significant animal and soon after effective completion of this phase the vaccine is prepared for human trials.

“In Phase 1, the age team profiling is done (whereby vaccine examine is finished on individuals in diverse age groups). In Period 2, approximately 600 to 700 individuals are vaccinated, and if the vaccine performs properly in this phase then it progresses into Phase 3, which is identified as the efficacy research (in this stage, 1000’s of folks are enrolled). Even just after rapidly tracking regulatory approvals, it will acquire at the very least 18 months to develop a great vaccine”, stated Ganguly.
He insisted that the COVID-19 vaccine remaining designed by Moderna (RNA vaccine is established to start Stage 3 trials later this month and targets a vaccine by 2021) and BioNTech-Pfizer (Section 3 trial is expected to commence in July). Gennova biopharmaceutical is also operating on a vaccine and it seems constructive, Ganguly included.

Musicaholic. Twitter guru. Total bacon fanatic. Zombie ninja. Freelance student. Coffee fan. Gamer.